Fundamentals of Batteries
Batteries are essential components of our modern world, powering everything from our smartphones and laptops to electric vehicles and grid-scale energy storage. Understanding the fundamentals of how batteries work is crucial for appreciating their role in our lives and for exploring the potential of new battery technologies.
Basic Principles of Battery Operation
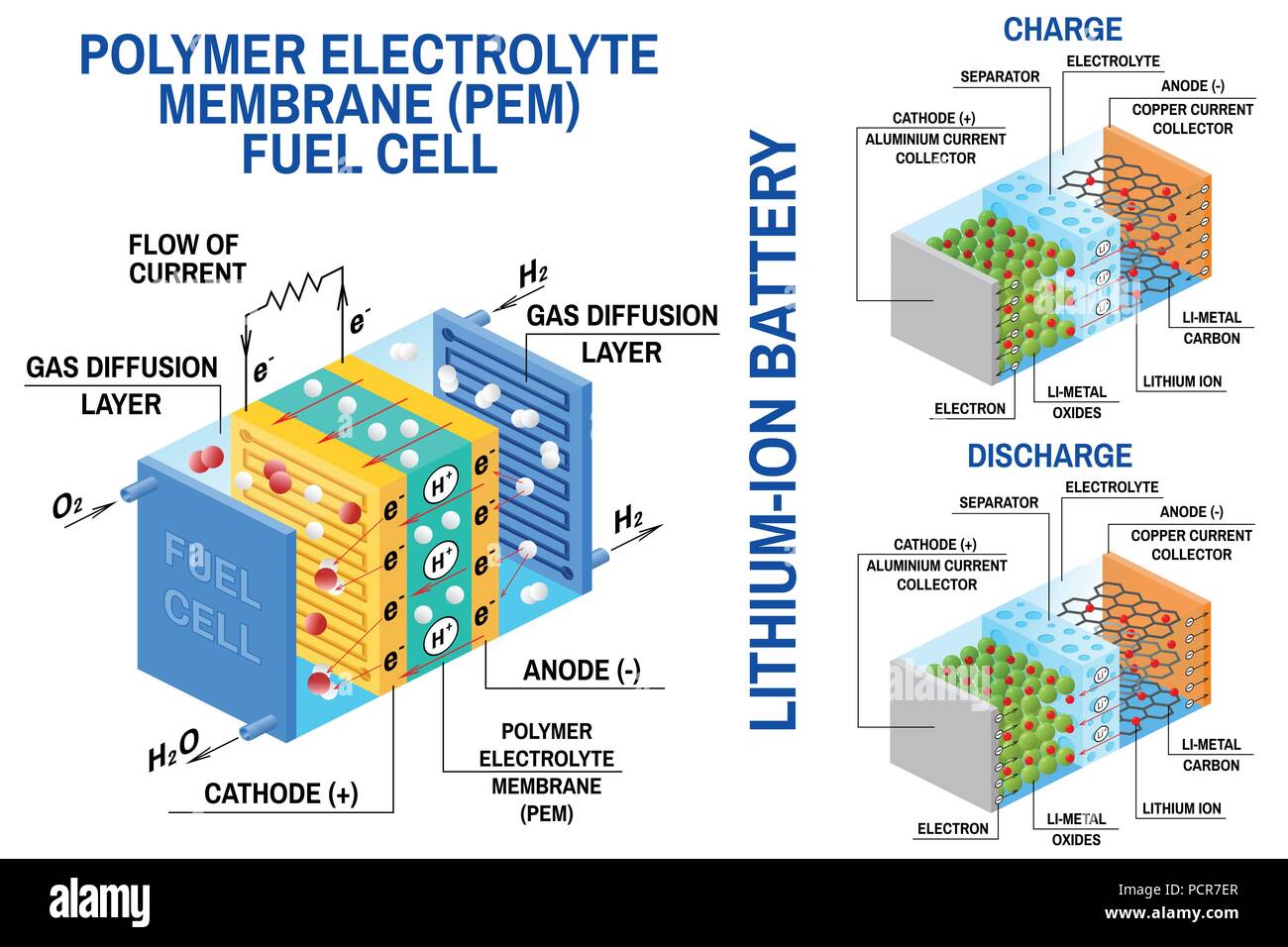
Batteries store energy through chemical reactions. The basic principle involves the conversion of chemical energy into electrical energy and vice versa. A battery consists of two electrodes, a positive electrode (cathode) and a negative electrode (anode), immersed in an electrolyte. When the battery is connected to a circuit, a chemical reaction occurs at the electrodes, causing electrons to flow from the anode to the cathode, generating an electric current. This flow of electrons is what powers devices connected to the battery.
Key Components of a Battery
- Electrodes: The electrodes are the sites where the chemical reactions occur. The anode is typically made of a material that easily releases electrons, while the cathode accepts electrons. The choice of materials for the electrodes significantly influences the battery’s performance and characteristics.
- Electrolyte: The electrolyte is a conductive solution that allows the movement of ions between the electrodes. It acts as a medium for the chemical reactions to take place. Different types of electrolytes are used depending on the battery chemistry and operating conditions.
- Separator: The separator is a porous membrane that prevents the electrodes from coming into direct contact, preventing short circuits. It allows the passage of ions but blocks the flow of electrons.
Types of Batteries
There are various types of batteries, each with its own advantages and disadvantages. Some common types include:
- Lead-Acid Batteries: These are the oldest and most widely used type of battery. They are relatively inexpensive and have a high discharge rate, making them suitable for applications such as car batteries and backup power systems. However, they are heavy and have a limited lifespan.
- Lithium-Ion Batteries: Lithium-ion batteries are known for their high energy density, lightweight, and long lifespan. They are widely used in portable electronics, electric vehicles, and energy storage systems. However, they can be expensive and have safety concerns related to thermal runaway.
- Alkaline Batteries: Alkaline batteries are commonly used in everyday devices like toys and remote controls. They offer a good balance of cost, performance, and shelf life. However, they have a lower energy density compared to lithium-ion batteries.
Comparison of Battery Characteristics
| Battery Type | Energy Density (Wh/kg) | Lifespan (Cycles) | Cost ($) |
|---|---|---|---|
| Lead-Acid | 30-40 | 300-500 | Low |
| Lithium-Ion | 150-250 | 500-1000 | Medium |
| Alkaline | 100-120 | 100-200 | Low |
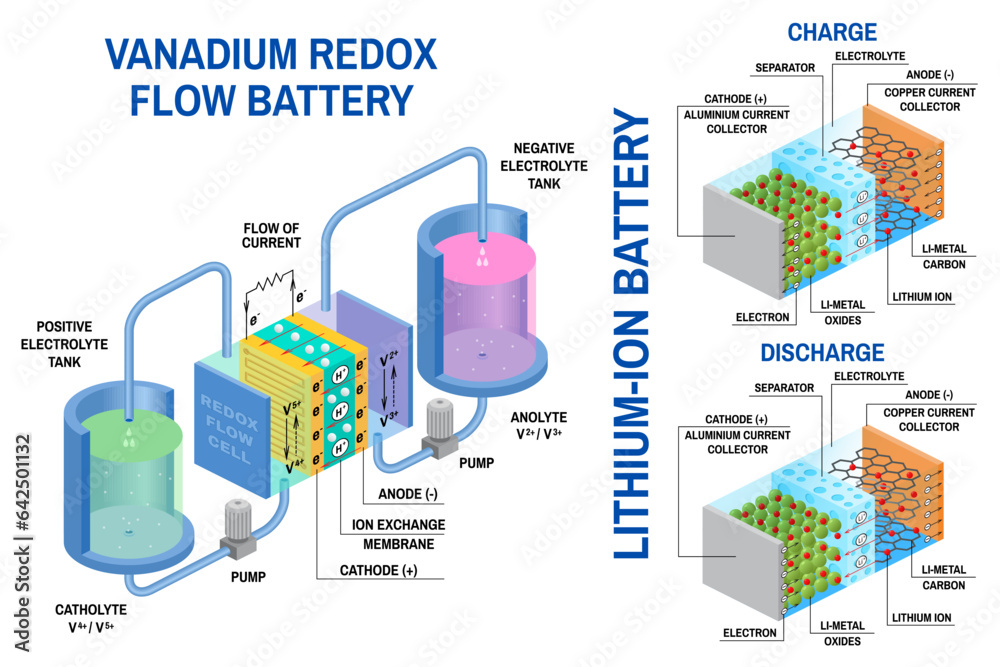
Battery Chemistry and Reactions: What Is Battery

Batteries operate based on electrochemical reactions, where chemical energy is converted into electrical energy and vice versa. Understanding these reactions is crucial to comprehending battery performance and limitations.
Electrochemical Reactions
Electrochemical reactions involve the transfer of electrons between chemical species, driving the flow of electric current. In a battery, these reactions occur at the electrodes, specifically at the anode (negative electrode) and cathode (positive electrode). During discharge, the anode undergoes oxidation, releasing electrons, while the cathode undergoes reduction, accepting electrons. This electron flow creates an electric current that can be used to power devices.
The specific reactions involved depend on the battery chemistry, but the general principle remains the same. For example, in a lithium-ion battery, the anode is typically made of graphite, which stores lithium ions (Li+), while the cathode is composed of a metal oxide, such as lithium cobalt oxide (LiCoO2). During discharge, lithium ions move from the anode to the cathode through an electrolyte, a conductive solution that allows the flow of ions but not electrons. This movement of ions is accompanied by the transfer of electrons from the anode to the cathode through an external circuit, generating electricity.
Role of Electrolytes and Electrodes
Electrolytes are crucial for battery operation, acting as a medium for ion transport between the electrodes. They are typically liquid solutions, but solid-state electrolytes are also being developed for improved safety and performance. Electrolytes must be chemically compatible with the electrodes and possess high ionic conductivity to facilitate efficient ion transport.
Electrodes, on the other hand, serve as the sites for the electrochemical reactions. They are typically made of materials with specific electrochemical properties, such as high conductivity and the ability to store and release ions reversibly. The choice of electrode materials significantly influences battery performance characteristics, such as capacity, voltage, and cycle life.
Battery Capacity
Battery capacity refers to the amount of charge that a battery can store. It is typically measured in ampere-hours (Ah) or milliampere-hours (mAh). The capacity of a battery is directly proportional to the amount of active material present in the electrodes. A higher amount of active material allows for the storage of more ions and, consequently, a larger charge capacity.
The capacity of a battery also depends on the battery chemistry and the design of the electrodes. For example, lithium-ion batteries with higher capacity are often achieved by using electrodes with larger surface areas or by employing materials with higher charge storage capabilities.
Battery Chemistry Flowchart
The following flowchart illustrates the fundamental processes involved in battery chemistry:
“`
+—————–+
| Battery Charging |
+—————–+
|
V
+—————–+
| Electrolyte |
+—————–+
|
V
+—————–+ +—————–+
| Anode (Oxidation) | | Cathode (Reduction) |
+—————–+ +—————–+
| |
V V
+—————–+ +—————–+
| Li+ Ions Flow | | Electron Flow |
+—————–+ +—————–+
| |
V V
+—————–+ +—————–+
| Stored Charge | | Electrical Energy |
+—————–+ +—————–+
|
V
+—————–+
| Battery Discharge |
+—————–+
“`
This flowchart depicts the key steps involved in battery charging and discharging. During charging, the external power source drives the flow of electrons from the cathode to the anode, forcing lithium ions to move from the cathode to the anode. This process stores charge within the battery. Conversely, during discharge, the flow of electrons is reversed, resulting in the release of electrical energy. The movement of ions and electrons is facilitated by the electrolyte and the electrochemical reactions at the electrodes.
Applications of Batteries
Batteries have become an indispensable part of modern life, powering a wide range of devices and systems. From the smallest portable electronics to large-scale energy storage systems, batteries play a crucial role in various industries, contributing significantly to technological advancements and sustainable energy solutions.
Applications in Electronics
Batteries are the primary power source for a vast array of electronic devices, including smartphones, laptops, tablets, and cameras. Their portability and convenience make them ideal for powering these devices on the go. Lithium-ion batteries, known for their high energy density and long lifespan, are commonly used in these applications.
- Smartphones and Tablets: Lithium-ion batteries provide the necessary power to run these devices for extended periods, enabling users to stay connected and productive throughout the day.
- Laptops and Notebooks: These devices rely on batteries to provide power for mobile computing, allowing users to work and access information from anywhere.
- Cameras and Camcorders: Batteries are essential for capturing images and videos, enabling photographers and videographers to document events and create content without being tethered to power outlets.
Applications in Automotive Industry
The automotive industry is witnessing a significant shift towards electric vehicles (EVs), and batteries are at the heart of this transformation. EV batteries store electrical energy, powering the electric motors that propel the vehicles. The range and performance of EVs are directly influenced by the capacity and efficiency of their batteries.
- Electric Vehicles (EVs): Batteries are the primary power source for EVs, enabling them to operate without relying on gasoline or diesel fuel. The development of high-capacity and long-lasting batteries has been crucial in driving the adoption of EVs.
- Hybrid Electric Vehicles (HEVs): HEVs combine a gasoline engine with an electric motor, using batteries to supplement the engine’s power and improve fuel efficiency. Batteries in HEVs are typically smaller than those in EVs, providing auxiliary power rather than primary propulsion.
Applications in Renewable Energy
Batteries play a vital role in the integration of renewable energy sources, such as solar and wind power, into the electricity grid. They provide a means to store excess energy generated from these sources, ensuring a reliable and consistent supply of electricity even when renewable energy generation is intermittent.
- Grid-Scale Energy Storage: Large-scale battery systems can store energy generated from solar and wind farms, releasing it when demand exceeds generation. This helps to stabilize the grid and ensure a reliable power supply.
- Residential Energy Storage: Homeowners can install battery systems to store solar energy generated from rooftop panels, reducing their reliance on the grid and lowering energy costs.
Advantages and Disadvantages of Battery Applications
Batteries offer several advantages in various applications, but they also come with certain disadvantages that need to be considered.
Advantages
- Portability: Batteries are compact and lightweight, making them ideal for powering portable devices.
- Clean Energy: Batteries do not produce emissions during operation, contributing to a cleaner environment.
- Energy Storage: Batteries can store energy generated from renewable sources, enabling a more reliable and sustainable energy system.
- Increased Efficiency: Batteries can improve the efficiency of various systems, such as electric vehicles and renewable energy installations.
Disadvantages
- Limited Lifespan: Batteries have a limited lifespan, eventually losing their capacity to store energy.
- Cost: Battery production and disposal can be expensive, particularly for large-scale applications.
- Safety Concerns: Some battery types, such as lithium-ion batteries, can pose safety risks if not handled properly.
- Environmental Impact: The extraction of raw materials for battery production and the disposal of spent batteries can have environmental consequences.
Comparison of Battery Applications, What is battery
Batteries are used in a wide range of applications, each with specific requirements and considerations.
Portable Devices
- Size and Weight: Batteries in portable devices are typically small and lightweight, prioritizing portability and convenience.
- Energy Density: These batteries need to have a high energy density to provide sufficient power for extended use.
- Lifespan: The lifespan of batteries in portable devices is crucial, as users expect these devices to function reliably for a reasonable period.
Electric Vehicles
- Capacity: EV batteries need to have a high capacity to provide sufficient range for driving long distances.
- Charging Time: The charging time for EV batteries is an important consideration, as users need to be able to recharge their vehicles efficiently.
- Safety: EV batteries must meet stringent safety standards to prevent accidents and ensure the safety of passengers.
Grid-Scale Energy Storage
- Capacity: Grid-scale battery systems require a large capacity to store significant amounts of energy for balancing supply and demand.
- Reliability: These batteries need to be highly reliable and durable to ensure a consistent power supply.
- Cost-Effectiveness: Grid-scale battery systems must be cost-effective to make them viable for large-scale deployment.
Visual Representation of Battery Applications
[Image Description: A visual representation showcasing the diverse applications of batteries in our daily lives. The image could depict a smartphone, a laptop, an electric vehicle, a solar panel connected to a battery storage system, and a wind turbine with a battery backup. This visual representation highlights the wide range of industries and sectors where batteries are playing a crucial role.]
What is battery – Think of a battery like a little energy pouch for your phone or laptop. It stores up that juice, ready to power your device. And speaking of power, you should check out Star Jackson , she’s a real inspiration! She’s like a battery for her community, always charging up positive vibes and making things happen.
So, yeah, batteries are pretty important, but sometimes, a little human energy is what really gets things going.
So, like, you know what a battery is, right? It’s that thing that powers your phone or your scooter. But did you know that Skai Jackson, the girl from that show “Jessie,” is now a total influencer? You can check out her latest video here , and see how she’s totally rocking the social media scene.
Anyway, back to batteries, they’re basically like the lifeblood of our modern world, right?